56+ how to calculate screen failure rate in clinical trials
Web Results There were a total of 583 successful Phase 1 enrollment and dose administration events out of 773 Phase 1 consent events 754 dose success rate. Web Our clinical trial recruitment rate calculator formula below shows how many randomized patients per month should come from each site your trial is working with.

Reduced Screen Failure Rates And Accelerated Enrollment A Case Study
Web The screen-failure rate of a clinical trial refers to the percent of subjects who undergo screening but do not meet the enrollment criteria of a trial and is a key.

. Thats nearly 2 higher than the average screen failure rate reported. Screen failure has a detrimental effect on trial. Many trials did not report on the numbers of and reasons.
Web Standardized screen failure data in clinical trials is hard to find and results across different types of studies are rarely published. ClearTrial Plan and Source Cloud Service leverages embedded industry intelligence and clinical knowledge to optimize your clinical study planning and. There are two main ways to do the calculation.
Web The implementation and utility of patient screening logs in a multicentre randomised controlled oncology trial. Web simple Excel formulas you can calculate for each site. Web Calculating RR per study is probably the most arguable process among feasibility and clinical research experts.
Web As of 2019 the average screen failure rate in global clinical trials stood at 363 across therapeutic areas. Web within a clinical trial setting eg acute vs. Web Contemporary trials in genitourinary cancer reported screen failure rates of approximately 20 to 30.
The main reasons again were. Web 2 Planning a Clinical Trial. Web Consequently strategies to reduce the screen failure rate are a high priority for clinical research organizations CROs and the companies developing NASH drugs.
To do this you. Web Among 14 phase 3 trials in kidney cancer 5 reported rates and the mean screen failure rate was 24 range 21-27. One review of clinical trials for.
Research-only investigative sites but pro-vides a framework for discussing which aspects of the. E number of days that have passed since their last screening e number of days that have passed since their last. Web One of the keys to running a successful clinical trial is the selection of high quality clinical sites ie sites that are able to enroll patients quickly engage them on an.

Screen Failure Rates Vs Number Of Patients Recruited Covance Download Scientific Diagram

Pk309a2ho51lhm

Screen Failure Rates Vs Number Of Patients Recruited Covance Download Scientific Diagram

Optimizing Screen Failures In Clinical Trials Proofpilot

Screen Failure Rates In Contemporary Randomized Clinical Phase Ii Iii Therapeutic Trials In Genitourinary Malignancies Sciencedirect
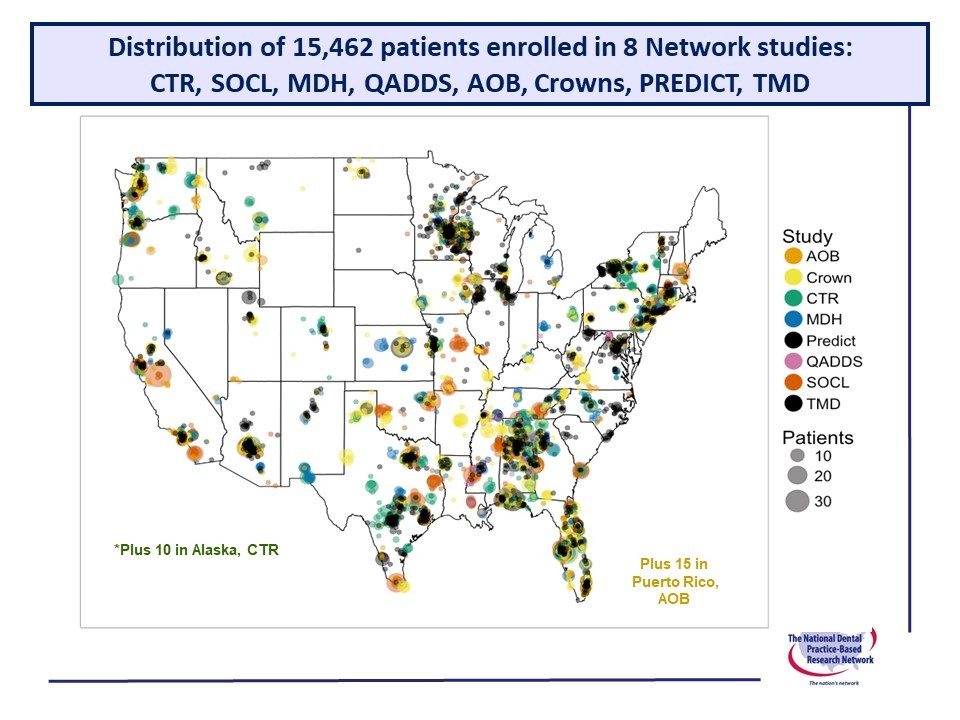
Recruiting For Studies Ongoing Upcoming Completed National Dental Pbrn
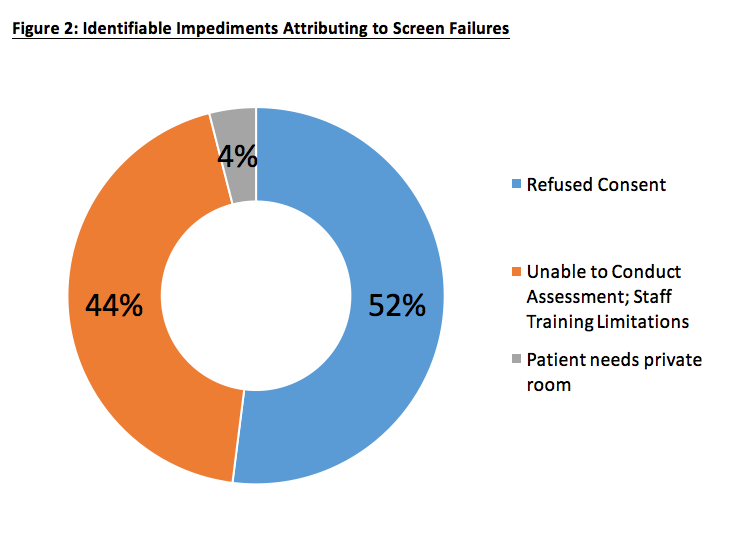
4 Steps To Mitigate Screen Failure Risks

3 Ways To Reduce Screen Failures During Clinical Trials

Screen Failure Rates In Contemporary Randomized Clinical Phase Ii Iii Therapeutic Trials In Genitourinary Malignancies Sciencedirect

Screen Failure In Clinical Trials Improving Reconciliation Advarra

A Multicenter Randomized Study Of Decitabine As Epigenetic Priming With Induction Chemotherapy In Children With Aml Biorxiv

Math6 Lm 2 Pdf Fraction Mathematics Numbers
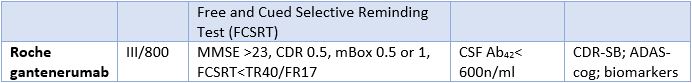
Unique 4 Step Approach Limits High Screen Failure In Alzheimer S Disease Research Part 1 Worldwide Clinical Trials
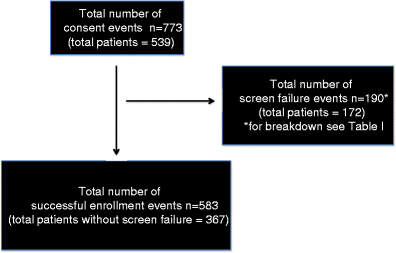
Determinants Of Patient Screen Failures In Phase 1 Clinical Trials Springerlink
4 Steps To Mitigate Screen Failure Risks

2020 Aapm R Annual Assembly Abstracts 2020 Pm Amp R Wiley Online Library
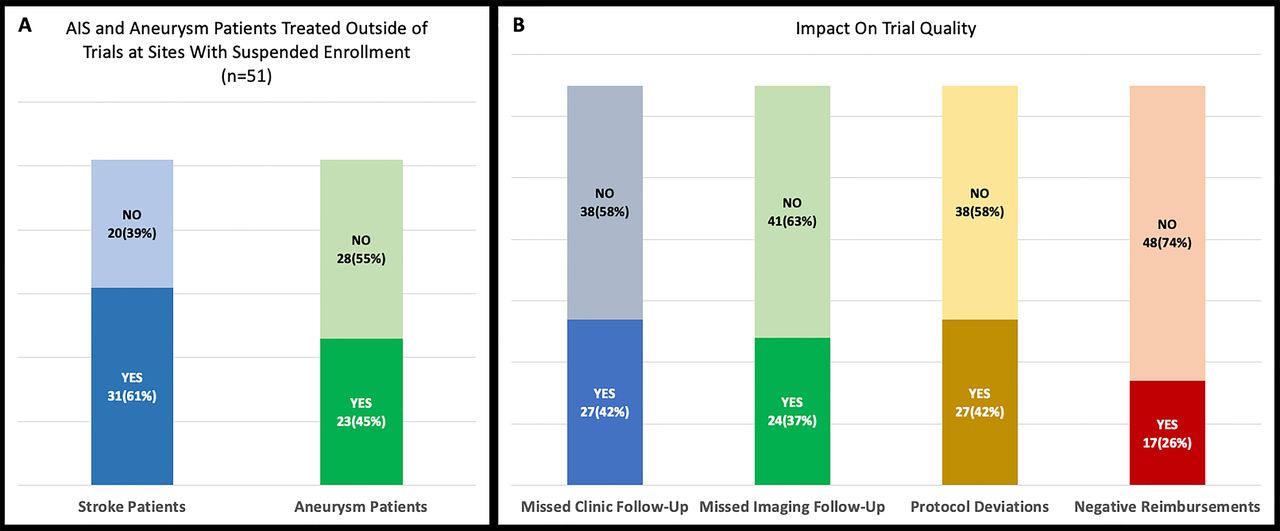
Neuroendovascular Clinical Trials Disruptions Due To Covid 19 Potential Future Challenges And Opportunities Journal Of Neurointerventional Surgery